Abstract
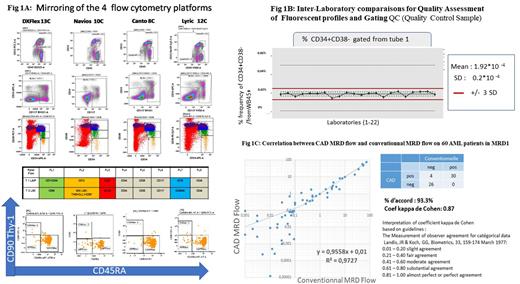
Introduction: MRD follow-up is now mandatory for treatment response evaluation in AML clinical trials (ELN 2017 and 2021 guidelines: Heuser et al,Blood doi:10.1182/blood.2021013626.). Beside to molecular biology methods, multiparameter flow cytometry assay represents the most reliable approach. The aim of this study was to implement an harmonized follow up of the patients using a standardized MRD flow approach across 30 French hematology laboratories participating in AML clinical trials. Methods: To obtain superposable results, the network has established recommendations from Wet labs procedure to clinical reports using common panels, flow cytometers settings and analysis strategy. We designed a 2-tubes panel with minimal mandatory 8c markers by tube, according to ELN recommendations to identify the pattern of LAIP/DfN in bulk cells and the LSC (Leukemia Stem Cells) in CD34+CD38- fraction. A « backbone » CD34/CD38/CD45/CD117 was used, completed by CD7,CD56, CD13, CD33, CD19 for the 1st tube and CD90(Thy-1), Mix (CD97+CLL1+TIM3), CD123, CD45RA for the 2nd one. This panel could be used in 8c,10c,12c (HLADR, CD36, CD10, CD200) implemented on Becton Dickinson (CANTO and LYRIC) or Beckman Coulter (NAVIOS and DxFlex) cytometers (Fig 1A). Harmonization of the sensitivity between platforms (18 BD and 12 BC) was performed using 8 Picks Rainbows calibration beads. Immunostaining was performed after "bulk" lysis technique (NH4Cl). A total of minimal 500 000 cells was acquired in each tube and data were analyzed using DIVA/FACSUITE and KALUZA software. Results: To detect any bias between the platforms, firstly we tested 10 EDTA fresh regenerative marrow samples in parallel at 2 platforms (CANTO and NAVIOS). Similarity of the staining index between positive and negative population were compared between the samples and the 2 platforms showing no differences. Secondly, regular QC (Quality Control Sample), of normal bone marrow (from healthy donor) 1/year (a total of 5 since 2017) was shared among the participants centers(Fig1B).The specific events populations (nHSC, MPP, LMPP"like,etc) were evaluated by centers using a common gating strategy. In a third step, standardization of the data analysis strategy obtained in the centers was subsequently evaluated using a virtual quality control(CQA) by sharing MRD FCS data files. About 90% of the locally analyzed data were included in an interval centralized on the average of the series mean +/- 2 SD. During the follow up of the patients, a series of 18 Web educational meeting was implemented to review and validate cases with difficulties regarding analysis interpretation and confrontation with molecular MRD (transcripts, NPM1, WT1, NGS) and also chimerism in allograft follow-up (mainly cases with clonal hematopoiesis or stressed bone marrow). Finally, the harmonized data generated in our group allowed to perform a Computer aided design (CAD) in assessment of AML MRD flow using proprietary developed R script based upon FlowSom. It performs an automated definition of metacluster with abnormal population (by comparison to a set of reference bone marrow) which are quantified in the CD34+ CD117+ space for all samples (diagnosis, follow up and references marrow). It then extracts the metacluster significantly different from that observed for the reference samples (> mean+6sd. It calculates the MRD in % of WBC CD45+. The limits of sensitivity of the CAD method shows that a MRD remains evaluable by CAD up to 0.04% (50 events in 1 000 000). We compared the results between the conventional and the CAD method on 60 MRD samples. The slope of the correlation line was 0.95 with an R2 of 0.97 (Fig 1C). Using the definition threshold of a positive MRD at 0.1%, the agreement of the results between the two methods was evaluated by the Cohen kappa coefficient of 0.87. Conclusions: This methodological validation protocol is a mandatory step to consider the use of MRD flow in AML clinical trials. Choosing a multicentric approach could be challenging, but our first results are promising and showed the feasibility of this concept when: (i) a straight harmonisation of the instruments sensitivity and samples preparation are established, and (ii) training and systematic education among the analytical operators are regularly performed. Finally, our pilot study shows a very strong correlation between conventional method and unsupervised analyses using data generated by the multicentric network.
Disclosures
Letestu:Abbvie: Consultancy, Other: Funding for congress expenses, Speakers Bureau; AstraZeneca: Speakers Bureau. Le Garff-Tavernier:Abbvie: Consultancy, Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal